

Glutathione beads incubated with: lane 3, GST-p21 supernatant and lane 4, GST supernatant. Thus, the interaction between PCNA and DNA. Protein A beads incubated with: lane 1, GST-p21 and lane 2, GST. In contrast, the loss of PCNA binding severely compromised the ability of DNA ligase I to join Okazaki fragments. After separation of proteins bound to beads by SDS/PAGE, PCNA was detected by immunoblotting with the PCNA monoclonal antibody. The protein A beads were removed by centrifugation, and the supernatants were incubated with glutathione beads. ( C) Equal amounts of the immunoprecipitated DNA ligase I–PCNA complex were incubated with GST (1 μg) or GST-p21 (1 μg). PCNA was detected in the immunoprecipitates by immunoblotting with the PCNA monoclonal antibody. ( B) DNA ligase (1 μg) and PCNA (0.5 μg) were preincubated as indicated and then immunoprecipitated with DNA ligase I antiserum as described in Materials and Methods. DNA ligase I and PCNA are indicated on the right. Proteins specifically bound to the beads or remaining in the supernatant were detected by immunoblotting with the polyclonal DNA ligase I antiserum and the PCNA monoclonal antibody after denaturing gel electrophoresis. ( A) DNA ligase I (180 ng) and PCNA (60 ng) were preincubated before incubation with either GST or GST-p21 beads as described in Materials and Methods. Interaction of the DNA ligase I–PCNA complex with GST-p21 beads. Thus, we propose that after Okazaki fragment DNA synthesis is completed by a PCNA-DNA pol delta complex, DNA pol delta is released, allowing DNA ligase I to bind to PCNA at the nick between adjacent Okazaki fragments and catalyze phosphodiester bond formation. Consistent with this idea, the cell cycle inhibitor p21, which also interacts with PCNA and inhibits processive DNA synthesis by DNA pol delta, disrupts the DNA ligase I-PCNA complex. These observations suggest that a ternary complex of DNA ligase I, PCNA and DNA pol delta does not form on a gapped DNA template. However, DNA ligase I inhibited PCNA-dependent DNA synthesis by DNA pol delta. An interaction between DNA ligase I and PCNA that is topologically linked to DNA was detected.
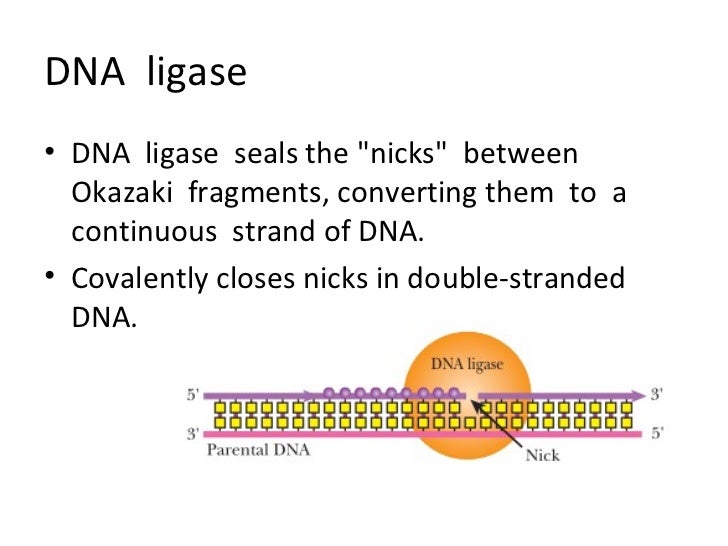
PCNA, which forms a sliding clamp around duplex DNA, interacts with DNA pol delta and enables this enzyme to synthesize DNA processively. Subsequent experiments demonstrated that DNA ligase I and PCNA interact directly via the amino-terminal 118 aa of DNA ligase I, the same region of DNA ligase I that is required for localization of this enzyme at replication foci during S phase. In this study, fractionation of a HeLa nuclear extract by DNA ligase I affinity chromatography resulted in the specific retention of a replication protein, proliferating cell nuclear antigen (PCNA), by the affinity resin. Although three human genes encoding DNA ligases have been isolated, the molecular mechanisms by which these gene products specifically participate in different DNA transactions are not well understood.


 0 kommentar(er)
0 kommentar(er)
